Author: [Poonamjeet Kaur]
Introduction
At the turn of the 20th century, our understanding of the atom was drastically reshaped by a groundbreaking experiment conducted by Ernest Rutherford. Prior to this, J.J. Thomson’s "plum pudding model" was widely accepted. But Rutherford’s gold foil experiment in 1909 changed everything.
The Gold Foil Experiment
In his famous experiment, Rutherford directed a stream of alpha particles at a very thin sheet of gold foil. Most of the particles passed straight through, but a few were deflected at large angles, and some even bounced back.
This unexpected outcome led to a new atomic model.
Rutherford’s Atomic Model: Key Features
1. Nucleus at the Center: The atom has a small, dense, positively charged center called the nucleus.
2. Electrons Orbit the Nucleus: Electrons revolve around the nucleus in circular paths, much like planets around the sun.
3. Mostly Empty Space: Most of the atom's volume is empty, allowing alpha particles to pass through the foil undisturbed.
Merits of Rutherford’s Model
Discovery of the Nucleus: For the first time, the atom was shown to have a central core—the nucleus.
Explained Scattering of Alpha Particles: The model successfully explained the results of the gold foil experiment.
Foundation for Modern Atomic Theory: It paved the way for Bohr’s model and quantum mechanics.
Demerits of Rutherford’s Model
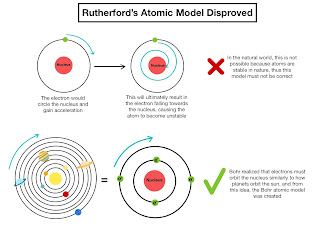
Instability of Electron Orbits: According to classical physics, revolving electrons should radiate energy and spiral into the nucleus—this doesn’t happen.
No Explanation for Atomic Spectra: It couldn’t explain the line spectra of elements, especially hydrogen.
No Energy Levels: It lacked the concept of quantized energy levels which was later introduced by Niels Bohr.
Conclusion
Rutherford’s model was a major leap forward in atomic theory. Despite its limitations, it laid the groundwork for more accurate models. His concept of a central nucleus remains one of the most vital contributions to atomic physics.
Thank You


Well written Blog , Easy to understand and Very Informative.
ReplyDeleteThanks Mr.Parveen for taking out time and reading the blog
DeleteVery Informative Blog
ReplyDeleteThanks Mr. Harry
Delete